20+ Calculate The Molality Of Phenol.
Web We have incorporated a reduced-form urban air chemistry model in the Massachusetts Institute of Technologys two-dimensional land and ocean resolving coupled chemistry-climate model. 20 aqueous solution of KI means 20 g of KI is present in 100 g of solution.

Molality Molarity Mole Fraction Numerical Problems
Web In this work a series of organic aromatic compounds was studied by various experimental and theoretical methods with the main objective of obtaining insights about the physical-chemical factors that might lead to structural and energetic differentiation among selected groups of interrelated molecules.

. Here students may access free resources on all the concepts that fall under the chemistry syllabus for classes 6-12. Web The mathematical techniques of abstract factor analysis AFA and target factor analysis TFA were used to study the role of molecular interactions on the retention mechanism of nitroanilines in normal phase high performance liquid chromatography. Calculate a molality b molarity and c mole fraction of KI if the density of 20 massmass aqueous KI solution is 1202 g mL-1.
To rank items as equivalent overlap them. Web In chemistry an acid dissociation constant also known as acidity constant or acid-ionization constant. Phenol C 6 H 5 OH is often used as an antiseptic in mouthwashes and throat lozenges.
Web Enter the email address you signed up with and well email you a reset link. It is the ratio of number of moles of a particular component to the total number of moles of the solution. Web What is the molality of the phenol in the solution.
If a mouthwash has a phenol concentration of 15. An alternative way to define the concentration of a solution is molality abbreviated m. Web Enter the email address you signed up with and well email you a reset link.
The solution in Figure 1211 contains 100 g of cobaltII chloride dihydrate CoCl 2 2H 2 O in enough ethanol to make exactly 500 mL of solution. Web Enter the email address you signed up with and well email you a reset link. Web The mathematical techniques of abstract factor analysis AFA and target factor analysis TFA were used to study the role of molecular interactions on the retention mechanism of nitroanilines in normal phase high performance liquid chromatography.
Calculate the molality of the solution. Out of molarity molality and mole fraction. Web A solution containing 20 g of phenol in 10 kg of benzene has its freezing point lowered by 069 K.
Denoted is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation in the context of acidbase reactionsThe chemical species HA is an acid that dissociates into A. That is 20 g of KI is present. 10 g glucose is dissolved in 400 g of solution.
Given K f for benzene 51 Km -1 Answer. To find the number of moles of CoCl 2 2H 2 O divide the. Which of the following is a dimensionless quantity.
An alternative way to define the concentration of a solution is molality abbreviated m. Mass of solute and volume of solution Asked for. Rank solutions from highest freezing point to lowest freezing point.
Calculate percentage concentration of the solution. Web -20 questions from exam 1 20 questions from exam 2 20 questions from exam three each 20 questions worth 20. Web The equation above can be used to calculate how much solute is required to make any amount of a desired solution.
Web The equation above can be used to calculate how much solute is required to make any amount of a desired solution. If the density of the solution is 1072 gml then what shall be the molarity of the solution. What is the molar concentration of CoCl 2 2H 2 O.
Web Molality of KI if the density of 20 massmass aqueous KI is 1202 gml. Web Solutions to problems P1C2 a Using the perfect gas law pV nRT the molar volume is calculate as RT 82057 102 dm3 atm K1 mol1 350 K Vm 125 dm3 mol1 p 230 atm. Calculate the fraction of phenol that has dimerised.
A It is due to formation of H-bonding due to which escaping tendency of molecules and vapour pressure of the solution decrease therefore boiling. A Molar mass of KI 39 127 166 g m o l 1 mol-1 m o l 1. For a solution containing n2 moles of the solute dissolved in n1 moles of the solvent 3.
Complete Solutions Manual GENERAL CHEMISTRY NINTH EDITION EbbingGammon. Associate professor tarikak nasserehab altimimi and thuria t mansoor Abstract. Web Welcome to the home page of the BYJUS chemistry e-library.
An alternative way to define the concentration of a solution is molality abbreviated m. Calculation of molality of solution Weight of KI in 100 g of the solution 20 g Weight of water in the solution 100 20 80 g 0-08 kg Molar mass of KI 39 127 166 g mol-1. Web The equation above can be used to calculate how much solute is required to make any amount of a desired solution.
Anew simple method for removal of Heavy metals Ions from terminal Wastewater using Iraqi Eucalyptus leaves Powder Tarik ak Nasser1ehab altimimiand thuria tMansoor2. Web The development of pharmaceutical technology in past years has presented the development of alternative dosage forms for patients who may have difficulty in swallowing of conventional tablets. Web Complete Solutions Manual General Chemistry Ninth Edition.
Web removal of heavy metals from food extract by adsorption on dried leaves Authors. Mole fraction is the dimensionless quantity as it is the ratio of similar quantities ie moles. 943 m A solution is made using 1115 g of dimethyl ether MM 4607 gmol and 900 g of methanol MM 3204 gmol.
Phenol C 6 H 5 OH is often used as an antiseptic in mouthwashes and throat lozenges. Molarity molality or mole fraction. It is obtained by using the following relation.
If a mouthwash has a phenol concentration of 15. Phenol C 6 H 5 OH is often used as an antiseptic in mouthwashes and throat lozenges. C3H8O3 0020 m KBr 0030 m phenol C6H5OH.
If a mouthwash has a phenol concentration of 15. Web a Molality of KI b Molarity of KI c Mole fraction of KI. Calculate the molality of ascorbic acid in this solution.
The Activated Sludge Ecosystem Contains A Core Community Of Abundant Organisms The Isme Journal

Math Physics Chemistry Questions Discussion Lists Dated 2015 06 20

Stick Representation Of The Different Tautomers Of D Glucose And Download Scientific Diagram

Pdf Conductometric Study Of Sodium Dodecyl Sulfate Nonionic Surfactant Triton X 100 Tween 20 Tween 60 Tween 80 Or Tween 85 Mixed Micelles In Aqueous Solution

Calculate The Molarity And Molality Of 20 Aqueous Ethanol C2h5oh Solution By Volume Density Of Solution 0 96 G Ml
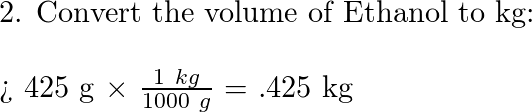
A Solution Is Made Containing 20 8 G Of Phenol C 6h 5o Quizlet

1dfl3o0ihcqc Pdf Pdf

Pdf Spectrophotometric Determination Of The Ionization Constants Of Aqueous Nitrophenols At Temperatures Up To 225 C

Calculate The Molality O 20 W W Soln Of Acetic Acid In Water Brainly In

Calculate The Molarity And Molality Of 20 Aqua Solution Ethanol Solution By Mass Per Volume Density Brainly In

A Solution Containing 20 Percent Acetic Acid By Mass In Water If The Density Of Solution At 20 C Is 1 2 G Cm 3 What Is Molarity At 20 C

Calcualate The Molarity And Molality Of 20 Aqueous Ehtanol C 5 H 5 Oh Solution By Volum Youtube
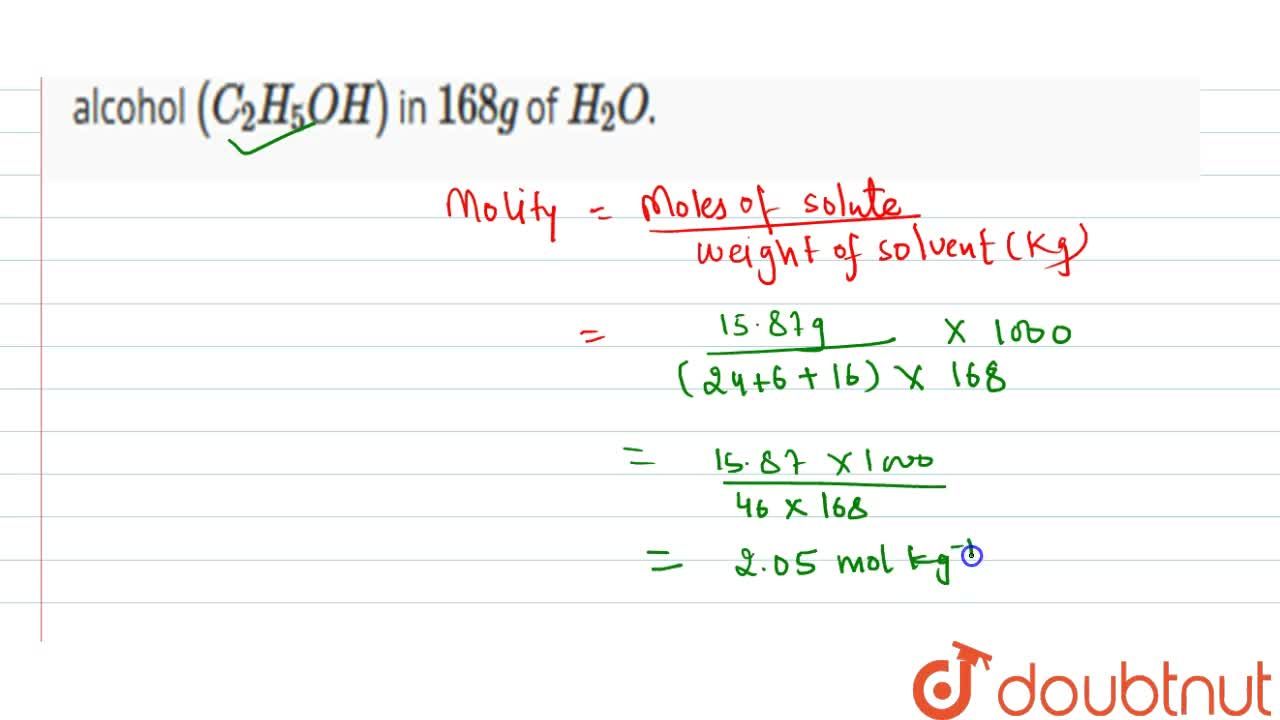
Calculate The Molality Of A Solution Obtained By Dissolving 15 87 G Ehtyl Alcohol C 2 H 5 Oh In 168 G Of H 2 O
Solved Course Hero

Physical Chemistry Chem 311

Calculate The Molarity And Molality Of 20 Aqua Solution Ethanol Solution By Mass Volume Density Of Brainly In

Calculate Molality Chemistry Questions